The secret to staying young? Scientists boost lifespan of mice by deleting defective cells
Richard Faragher, University of Brighton
The ageing population is one of the greatest challenges facing society. More people are surviving to old age than ever before, but we currently lack the means to keep them healthy and independent. If a treatment existed to reduce sickness and death from ageing by 20% then between now and 2050, the US alone would save US$4 trillion on healthcare costs – enough money to give everyone on Earth clean drinking water for the next three decades.
However a landmark new study, published in Nature, raises hopes that such a treatment will be possible. The researchers managed to increase the lifespan of mice by an impressive 25% by deleting “senescent” cells, dysfunctional cells which build up as we age and cause damage to tissue. Crucially, the mice lived longer because they were healthier.
Ageing versus disease
Unless you are exceptionally thoughtful, unusually well informed or a bio-gerontologist (a specialist in the biology of ageing), everything you think you know about the relationship between the ageing process and diseases such as cancer or atherosclerosis is probably wrong.
We have always known that those around us will grow old, get sick and die. But few of us have stopped to think about how this actually happens. What is the relationship between “natural changes” like wrinkles and “diseases” that can actually kill us?
Greek doctor and philosopher Aelius Galen (c. 121-169 AD) set the conceptual framework for our understanding of ageing. He defined disease as an abnormal function. Since ageing is universal it cannot, he reasoned, be a disease by definition. Although there were later variations on this argument, they lead to the same conclusion. If ageing takes place in everyone and disease occurs in only a part of the population then disease and ageing are not synonymous. The former should be cured and the latter endured, or perhaps celebrated, went the conventional wisdom.
Y tambe/wikimedia, CC BY-SA
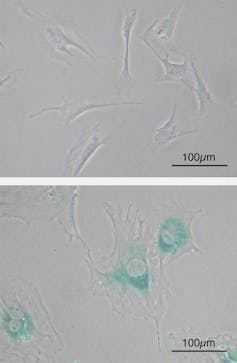
But the people making these arguments had no meaningful idea of the mechanisms that cause either ageing or disease. It wasn’t until the 1980s that researchers started to really understand the biology of ageing. One hypothesis that emerged is that the accumulation of “senescent” cells may be a driving force of ageing.
Senescent cells are formed within the cell populations that divide during life. However, this division is limited as an anti-cancer mechanism and so after a variable degree of replication, cells stop dividing and enter the senescent state.
Once senescent these cells produce a range of inflammatory molecules and undergo other changes which damage tissue. These changes alert the immune system to their presence allowing it to remove them. Unfortunately as the immune system itself ages this capacity declines and the number of senescent cells in tissue increases, leading to ageing.
Evidence at last?
A pretty story, but most of the underpinning research was generated in the abnormal environment of the tissue culture dish. For this reason many researchers were sceptical, asking for the real proof.
And the new study provides just that. The researchers genetically engineered mice so that their senescent cells could undergo programmed cell death if treated with a small molecule. The results of deleting senescent cells in this manner are impressive. Median lifespan increased by about 25%. This is a similar effect to that of two laboratory interventions already known to extend healthy lifespan in mice – dietary restriction and supplementation with the drug rapamycin.
The animals showed reduced deterioration of several organ systems and delayed development of cancers – without side-effects. The authors of the paper were rightly both excited and modest in pointing out the prospect that deleting senescent cells could eventually extend healthy human lifespan.
There are two ways to achieve this and groups are already working on both. The first of these is to identify drugs which kill senescent cells, and the second is to develop drugs which block their effects. Both have promise but perhaps a combination of the two has the most promise of all. However, the road to the clinic can be long. Although tamoxifen is now almost a household name as a breast cancer treatment, it was first synthesised in 1962 but not shown to be beneficial until 1972.
Perhaps most importantly, work on senescent cells illustrates that the distinction between ageing and age-related disease, held for so long, is a false dichotomy. Senescent cells in the skin are responsible for wrinkles, a “natural change”, and for cardiovascular disease, “an age-related disease”.
![]() Instead of “ageing” and “disease” mechanisms, mammals have key health maintenance mechanisms and problems start when these fail. Knowing what they are opens the prospect of broad preventative treatments that will allow us to live well and hopefully to live longer too.
Instead of “ageing” and “disease” mechanisms, mammals have key health maintenance mechanisms and problems start when these fail. Knowing what they are opens the prospect of broad preventative treatments that will allow us to live well and hopefully to live longer too.
Richard Faragher, Professor of Biogerontology, University of Brighton
This article was originally published on The Conversation. Read the original article.



