Regrowing knee cartilage: new animal studies show promise
Suzanne E Eldridge, Queen Mary University of London; Anne-Sophie Thorup, Queen Mary University of London, and Francesco Dell’Accio, Queen Mary University of London
In our joints, a slippery, elastic tissue called cartilage covers the ends of the articulating bones. If this protective covering is lost through injury or ageing, it leaves bone grinding painfully against bone.
Cartilage injuries are very common and rarely heal spontaneously. This can cause further damage to the joint, leading to osteoarthritis – one the most common type of arthritis and a leading cause of disability worldwide. Between 10% and 15% of all people over the age of 60 have some degree of osteoarthritis. Unfortunately, there is no cure – but help may be on the way.
Our research group at Queen Mary, University of London, has developed two ways to encourage cartilage healing and improve joint pain in animals with osteoarthritis. We have also shown that these methods work on human cartilage cells in a test tube.
Agrin: a healing molecule
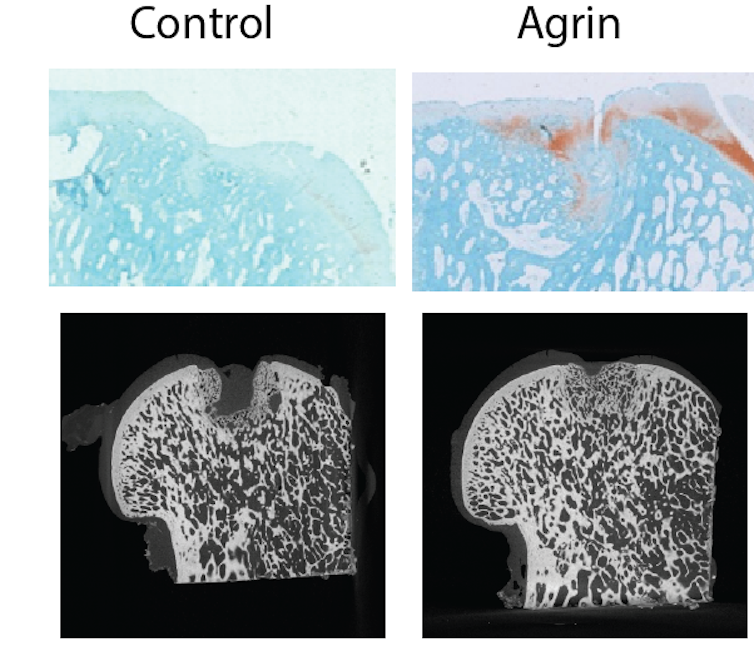
We discovered that a molecule called agrin can be used to treat very large injuries to the cartilage and bone. When agrin is implanted in cartilage and bone injuries, it quickly activates dormant stem cells in the joint and instructs them to repair the injury.
We performed our studies in mice and sheep. In sheep, a single administration of agrin into the cartilage defect was enough to trigger repair and increase the activity of the sheep for the six-month duration of the study.
There are other methods that have been developed to make new cartilage from cartilage cells or stem cells, but they involve taking the stem cells out of the patients’ knees, growing and treating them in the laboratory with various substances to induce them to make cartilage, and implanting them back into the joint.
This is laborious, costly, and has inconsistent results. Our approach skips all these steps. The stem cells are already there, in the knee. We have learned a way to “talk to them” using a drug and get them to do their job efficiently without having to take them out of the body.
Author provided
Unlike some other molecules that have been tested, agrin does not induce cartilage and bone to grow in places where it is undesirable. Agrin reactivates processes in adults that are used to shape the joint when we develop in the womb. We think this is why individual cells in the damaged knee get to know whether to make cartilage or bone – and where to make them.
While we didn’t notice any obvious side-effects in the mice and sheep – in fact, the sheep receiving agrin were much more active for the duration of the study, suggesting pain relief – more extensive animal studies are necessary before studies in humans are possible.
Another important limitation is that our studies were done in recent injuries and in relatively young animals, so we still don’t know if this approach will work in older people or in old knee injuries.
Blocking ROR2
In another study, we found that a molecule called ROR2 is absent in healthy cartilage but is produced after injury and contributes to cartilage breakdown in osteoarthritis. We wondered if blocking ROR2 would help alleviate osteoarthritis.
Whereas the studies with agrin were to treat a simple hole in the cartilage in an otherwise healthy knee, we tested a different molecule in mice whose knees had been made unstable – as you would have with a cruciate ligament tear. This led to widespread, severe wear and tear of the cartilage.
We managed to stop cartilage cells producing ROR2, using gene-silencing technology called “small interfering RNA”. We saw that the cartilage was protected to some degree from wearing down further. There was also rapid and clear pain relief, too rapid to be due to the protection of cartilage from degradation, which takes much longer.
We also saw improved cartilage formation in test tubes with human cartilage cells. However, before we can perform studies in humans using the ROR2 blockade approach, we need to repeat the studies in large animals such as sheep, perform more toxicity studies and we also need to make our molecule more stable so that we don’t need to inject it too often.
The hard work is starting to pay off
For both approaches, we are still a long way from having a treatment that can be used in clinical practice, but, if all goes well, we could be in clinical trials in a few years.
Our aim is to turn osteoarthritis into a curable or at least preventable disease. If this approach works in humans, we expect that a simple knee injection or some keyhole surgery will be enough to heal cartilage defects and prevent further damage. We might even be able to treat already developed osteoarthritis and avoid the need for joint replacement.
It’s disheartening having to tell patients with osteoarthritis that there is little we can do for them except offer painkillers and physiotherapy. It is a dream to be able to offer a medical treatment that can return these patients to their jobs, to their hobbies and to fulfilling lives. We are hopeful that all the efforts to understand how the joint cells work are finally paying off, and we are near to achieving this dream.![]()
Suzanne E Eldridge, Lecturer of Musculoskeletal Regenerative Medicine, Queen Mary University of London; Anne-Sophie Thorup, Postdoctoral Research Fellow in Experimental Medicine and Rheumatology, Queen Mary University of London, and Francesco Dell’Accio, Professor of Musculoskeletal Regenerative Medicine & Rheumatology, Queen Mary University of London
This article is republished from The Conversation under a Creative Commons license. Read the original article.